dr. d.'s projects
 |
Bottlenose dolphins (Tursiops truncatus) can exhale and inhale in 0.6 seconds. They then hold their breath for one to two minutes while they are at rest, or for periods up to eight minutes while they are diving. I am interested in determining which muscles power this unique combination of an explosive exchange of respiratory gases with periods of breath-hold. To identify the breathing muscles of dolphins, I am investigating muscles that have been implicated in the breathing mechanics of terrestrial mammals - diaphragm and scalenus. |

|
In addition, I looked at the fiber-type profile of the dolphin diaphragm using histochemical and biochemical techniques and found this muscle to be primarily composed of slow-twitch fibers. This characteristic does not seem to fit with the quick inhalation of dolphins. However, the construction of the dolphin diaphragm suggests that it acts as a spring, being loaded during exhalation and recoiling caudally to cause inhalation. In addition, two of the myosin isoforms expressed by this muscle are different from those found in rat diaphragm. To date, the contractile abilities of these unique myosins are unknown, but they may allow the dolphin diaphragm to contract more quickly than its histochemical profile would suggest. |
|
Being born directly into the aquatic environment presents unique challenges for the breathing muscles of neonatal dolphins and manatees. Not only must these muscles function from the moment of birth to allow gas exchange, but their activities must also be coordinated such that breathing occurs at the surface. However, the breathing muscles of dolphin and manatee neonates are able to deal with these demands. Thus, I hypothesize that these muscles are well-developed at birth, as demonstrated by the histochemical and biochemical activities of their component muscle fibers. |
 |

|
I completed a study of the neonatal dolphin diaphragm, and the results of this study are now available in the Journal of Morphology. Check out the abstract! |
|
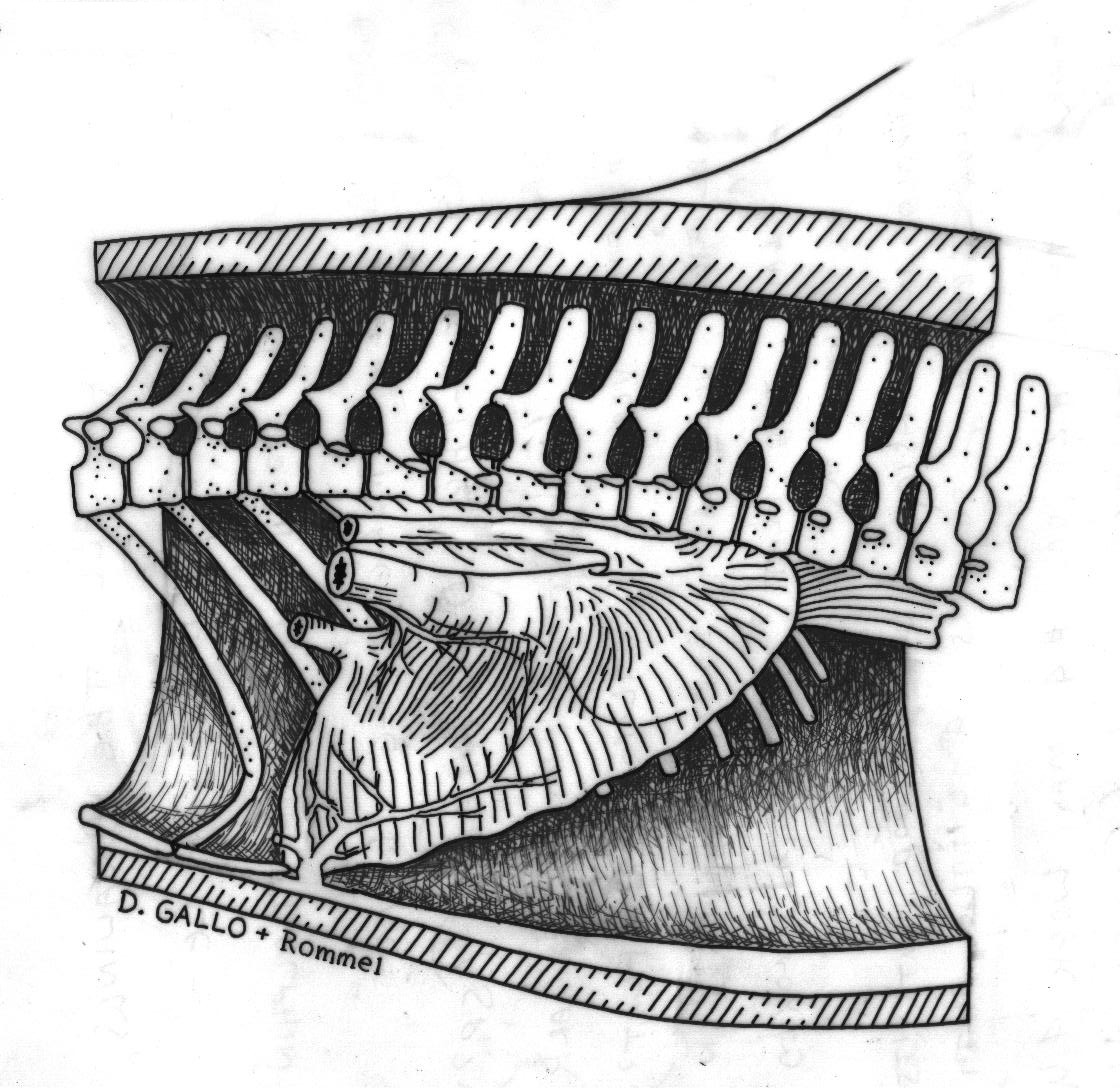
In the manatee ( Trichechus manatus latirostris), I am working to characterize the morphology and innervation pattern of the diaphragm. The diaphragm in these animals is a huge muscle, running almost the entire length of the body cavity. I am interested in determining if this muscle is "compartmentalized", i.e. having distinct regions that could possibly be recruited individually. If this muscle is "compartmentalized," the manatee would be able to contract distinct portions of its diaphragm, rather than having the entire muscle contract as a unit. Thus, as Rommel and Reynolds (2000) suggest, the manatee would be able to control the amount of air in different areas of its lungs, allowing it to determine its pitch in the water column. |
 |

|
Detailed dissections of the manatee diaphragm have revealed vascular connective tissue (VCT) bundles that may be involved in compartmentalizing this muscle. These elements are spaced approximately one centimeter apart down the length of the diaphragm (cranial to caudal) and extend through the depth of the muscle (abdominal to thoracic). Thus, the VCT bundles separate the muscle of the diaphragm into one centimeter wide blocks. However, the innervation pattern of the manatee diaphragm suggests that the actual compartments are made up of groups of these muscle blocks. Currently, I am working to identify the vascular elements of the VCT bundles using histology, and I am also characterizing the fiber-type profile of the compartments. |
 Return to research page
Return to research page  Return to home page
Return to home page